
Specific Latent Heat
A change of state requires energy
When a substance is melting or boiling, you’re still putting in energy and so increasing the internal energy, but the energy’s used for breaking intermolecular bonds rather than raising the temperature. There are flats spots on the heating graph where energy is being transferred but not being used to change the temperature.

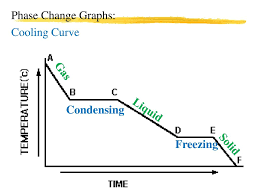
When a substance is condensing or freezing, bonds are forming between particles which releases energy. This means the internal energy decreases, but the temperature doesn’t go down until all the substances has turned to liquid or a solid. The flat parts of the graph show this energy transfer.
The energy needed to change the state of a substance is called latent heat
Specific latent heat is the energy needed to change the state of a 1kg matter
The specific latent heat of a substance is the amount of energy needed to change 1kg of it from one state to another without changing its temperature
For cooling, specific latent heat is the energy released by a change in state
Specific latent heat is different for different materials, and for changing between different states
The specific latent heat for changing between a solid and liquid is called the specific latent heat of fusion. The specific latent heat for changing between a liquid and a gas is called the specific latent heat of vaporization
There’s a formula for specific latent heat
You can work out the energy needed when a substance of mass m changes state using this formula
Energy = Mass x Specific latent heat
Energy is given in joules, mass in kg and SLH in J/kg
Specific Latent Heat
A change of state requires energy
When a substance is melting or boiling, you’re still putting in energy and so increasing the internal energy, but the energy’s used for breaking intermolecular bonds rather than raising the temperature. There are flats spots on the heating graph where energy is being transferred but not being used to change the temperature.

When a substance is condensing or freezing, bonds are forming between particles which releases energy. This means the internal energy decreases, but the temperature doesn’t go down until all the substances has turned to liquid or a solid. The flat parts of the graph show this energy transfer.

The energy needed to change the state of a substance is called latent heat
Specific latent heat is the energy needed to change the state of a 1kg matter
The specific latent heat of a substance is the amount of energy needed to change 1kg of it from one state to another without changing its temperature
For cooling, specific latent heat is the energy released by a change in state
Specific latent heat is different for different materials, and for changing between different states
The specific latent heat for changing between a solid and liquid is called the specific latent heat of fusion. The specific latent heat for changing between a liquid and a gas is called the specific latent heat of vaporization
There’s a formula for specific latent heat
You can work out the energy needed when a substance of mass m changes state using this formula
Energy = Mass x Specific latent heat
Energy is given in joules, mass in kg and SLH in J/kg
 Knowt
Knowt
