
Chapter 6: Ionic and Molecular Compounds
6.1: Ions: Transfer of Electrons
Most of the elements, except the noble gases, are found in nature combined as compounds.
One explanation for the stability of noble gases is that they have a filled valence electron energy level.
In the formation of either an ionic bond or a covalent bond, atoms lose, gain, or share valence electrons to acquire an octet of eight valence electrons.
Octet Rule: The tendency of atoms to attain a stable electron arrangement provides a key to our understanding of the ways in which atoms bond and form compounds.
Ionic Bonds: Occurs when the valence electrons of atoms of a metal are transferred to atoms of nonmetals.
Covalent Bonds: Occurs when atoms of nonmetals share valence electrons.
Positive Ions
Positive Ions: The loss of electrons.
In ionic bonding, ions form when atoms lose or gain electrons to form a stable electron arrangement.
Ionic Charge: A positive electrical charge.
Cations: Positively charged ions.
Negative Ions
Negative Ions: The gain of electrons.
In an ionic compound, a nonmetal atom gains one or more valence electrons to obtain a stable electron configuration.
Anion: A negatively charged ion.
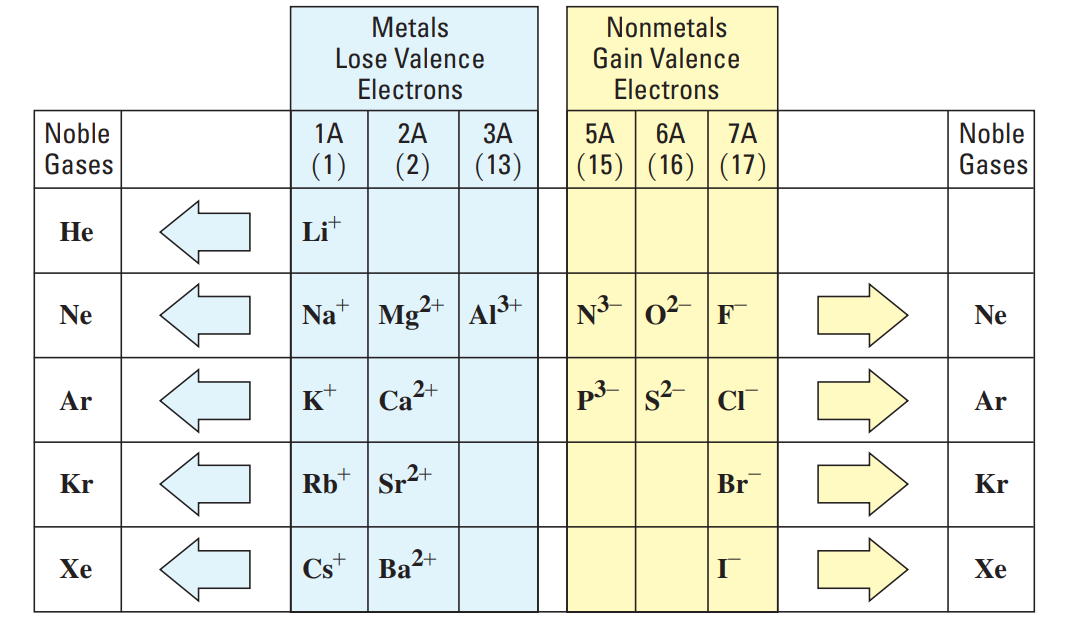
Ionic Charges from Group Numbers
The elements in Group 1A lose one electron to form ions with a 1+ charge.
The elements in Group 2A lose two electrons to form ions with a 2+ charge.
The elements in Group 3A lose three electrons to form ions with a 3+ charge.
The elements in Group 7A gain one electron to form ions with a 1– charge.
The elements in Group 6A gain two electrons to form ions with a 2– charge.
The elements in Group 5A gain three electrons to form ions with a 3– charge.
The nonmetals of Group 4A 1142 do not typically form ions.
The metals Sn and Pb in Group 4A lose electrons to form positive ions.
6.2: Ionic Compounds
Ionic compounds consist of positive and negative ions.
Ionic Bonds: The ions are held together by strong electrical attractions between the oppositely charged ions.
The positive ions are formed by metals losing electrons, and the negative ions are formed when nonmetals gain electrons.
The chemical formula of a compound represents the symbols and subscripts in the lowest whole-number ratio of the atoms or ions.
The subscripts in the formula of an ionic compound represent the number of positive and negative ions that give an overall charge of zero.
Formula Unit: The lowest ratio of the ions in an ionic compound.
6.3: Naming Ionic Compounds
In the name of an ionic compound made up of two elements, the name of the metal ion, which is written first, is the same as its element name.
The name of the nonmetal ion is obtained by using the first syllable of its element name followed by –ide.
Examples: Potassium iodide, Magnesium bromide & Aluminum oxide
6.4: Polyatomic Ions
Polyatomic ion: A group of covalently bonded atoms that has an overall ionic charge.
Most polyatomic ions consist of a nonmetal such as phosphorus, sulfur, carbon, or nitrogen covalently bonded to oxygen atoms.
The names of the most common polyatomic ions end in –ate, such as nitrate and sulfate.
When a related ion has one less oxygen atom, the –ite ending is used for its names such as nitrite and sulfite.
Note that both the –ate ion and –ite ion of a particular nonmetal have the same ionic charge.
6.5: Molecular Compounds: Sharing Electrons
Molecular Compound: It contains two or more nonmetals that form covalent bonds.
When atoms share electrons, the bond is a covalent bond.
When two or more atoms share electrons, they form a molecule.
Lewis Structure
The shared electrons, or bonding pairs, are shown as two dots or a single line between atoms.
The nonbonding pairs of electrons, or lone pairs, are placed on the outside.
Double Bond: It occurs when two pairs of electrons are shared.
Triple Bond: It occurs when three pairs of electors are shared.
Double and triple bonds form when the number of valence electrons is not enough to complete the octets of all the atoms in the molecule.
6.6: Electronegativity and Bond Polarity
The electronegativity of an atom is its ability to attract the shared electrons in a chemical bond.
Nonmetals have higher electronegativities than metals because nonmetals have a greater attraction for electrons than metals.
Fluorine (F) has the highest electronegativity which has a value of 4.0.
Metal Cesium (Cs) has the lowest electronegativity which has a value of 0.7.
The noble gases have no electronegativity values because they do not typically form bonds.
Nonpolar Covalent Bonds: A bond between atoms with identical or very similar electronegativity values.
Polar Covalent Bond: Occurs when bonds are between atoms with different electronegativity values, and the electrons are shared unequally.
Dipole: A polar covalent bond that has a separation of charges.
6.7: Shapes and Polarity of Molecules
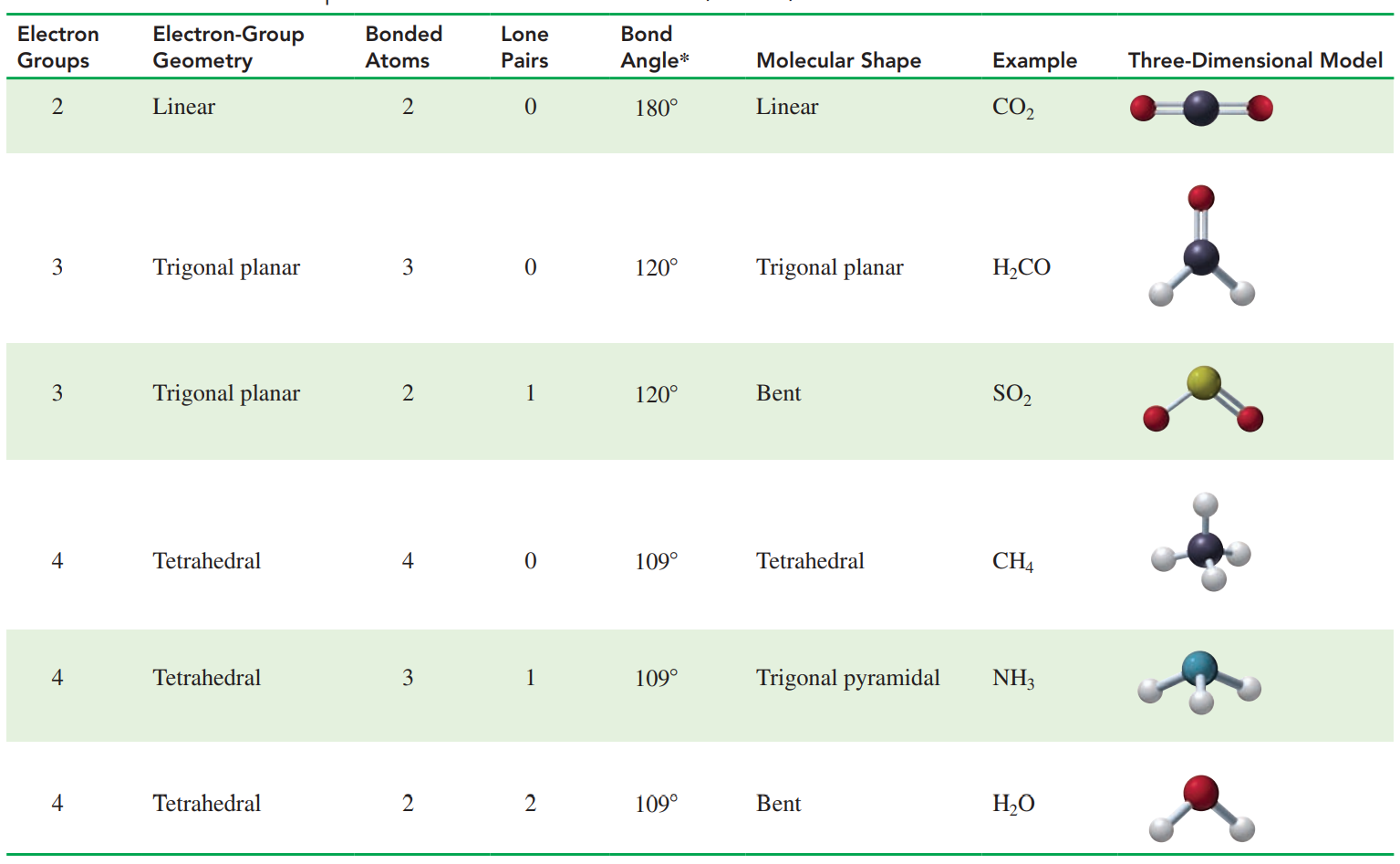
Valence shell electron-pair repulsion (VSEPR) theory: The electron groups are arranged as far apart as possible around the central atom to minimize the repulsion between their negative charges.
According to VSEPR theory, minimal repulsion occurs when two electron groups are on opposite sides of the central Carbon (C) atom.
This gives the Carbon Dioxide (CO2) molecule a linear electron-group geometry and a linear shape with a bond angle of 180°.
Minimal repulsion occurs when three electron groups are as far apart as possible around the central C atom, which gives 120° bond angles.
This type of electron-group geometry is trigonal planar.
When there are four atoms attached to four electron groups, the shape of the molecule is tetrahedral.
Molecular Shapes for a Central Atom with Two, Three, and Four Bonded Atoms
Polarity of Molecules
Nonpolar Molecule
All the bonds are nonpolar or the polar bonds cancel each other out.
It also occurs when polar bonds cancel each other because they are in a symmetrical arrangement.
Polar Molecules
One end of the molecule is more negatively charged than the other end.
It occurs when the dipoles from the individual polar bonds do not cancel each other.
6.8: Attractive Forces in Compounds
Dipole-Dipole Attractions: These are attractive forces that occur between the positive end of one molecule and the negative end of another.
Hydrogen Bonds: These occur between the partially positive hydrogen atom in one molecule and the partially negative nitrogen, oxygen, or fluorine atom in another molecule.
Polar molecules containing hydrogen atoms bonded to highly electronegative atoms of nitrogen, oxygen, or fluorine form especially strong dipole–dipole attractions.
Dispersion Forces: These are very weak attractions that occur between nonpolar molecules.
The electrons in a nonpolar covalent molecule are distributed symmetrically.
Electrons may accumulate more in one part of the molecule than another, which forms a temporary dipole.
Chapter 6: Ionic and Molecular Compounds
6.1: Ions: Transfer of Electrons
Most of the elements, except the noble gases, are found in nature combined as compounds.
One explanation for the stability of noble gases is that they have a filled valence electron energy level.
In the formation of either an ionic bond or a covalent bond, atoms lose, gain, or share valence electrons to acquire an octet of eight valence electrons.
Octet Rule: The tendency of atoms to attain a stable electron arrangement provides a key to our understanding of the ways in which atoms bond and form compounds.
Ionic Bonds: Occurs when the valence electrons of atoms of a metal are transferred to atoms of nonmetals.
Covalent Bonds: Occurs when atoms of nonmetals share valence electrons.
Positive Ions
Positive Ions: The loss of electrons.
In ionic bonding, ions form when atoms lose or gain electrons to form a stable electron arrangement.
Ionic Charge: A positive electrical charge.
Cations: Positively charged ions.
Negative Ions
Negative Ions: The gain of electrons.
In an ionic compound, a nonmetal atom gains one or more valence electrons to obtain a stable electron configuration.
Anion: A negatively charged ion.
Ionic Charges from Group Numbers
The elements in Group 1A lose one electron to form ions with a 1+ charge.
The elements in Group 2A lose two electrons to form ions with a 2+ charge.
The elements in Group 3A lose three electrons to form ions with a 3+ charge.
The elements in Group 7A gain one electron to form ions with a 1– charge.
The elements in Group 6A gain two electrons to form ions with a 2– charge.
The elements in Group 5A gain three electrons to form ions with a 3– charge.
The nonmetals of Group 4A 1142 do not typically form ions.
The metals Sn and Pb in Group 4A lose electrons to form positive ions.

6.2: Ionic Compounds
Ionic compounds consist of positive and negative ions.
Ionic Bonds: The ions are held together by strong electrical attractions between the oppositely charged ions.
The positive ions are formed by metals losing electrons, and the negative ions are formed when nonmetals gain electrons.
The chemical formula of a compound represents the symbols and subscripts in the lowest whole-number ratio of the atoms or ions.
The subscripts in the formula of an ionic compound represent the number of positive and negative ions that give an overall charge of zero.
Formula Unit: The lowest ratio of the ions in an ionic compound.
6.3: Naming Ionic Compounds
In the name of an ionic compound made up of two elements, the name of the metal ion, which is written first, is the same as its element name.
The name of the nonmetal ion is obtained by using the first syllable of its element name followed by –ide.
Examples: Potassium iodide, Magnesium bromide & Aluminum oxide
6.4: Polyatomic Ions
Polyatomic ion: A group of covalently bonded atoms that has an overall ionic charge.
Most polyatomic ions consist of a nonmetal such as phosphorus, sulfur, carbon, or nitrogen covalently bonded to oxygen atoms.
The names of the most common polyatomic ions end in –ate, such as nitrate and sulfate.
When a related ion has one less oxygen atom, the –ite ending is used for its names such as nitrite and sulfite.
Note that both the –ate ion and –ite ion of a particular nonmetal have the same ionic charge.
6.5: Molecular Compounds: Sharing Electrons
Molecular Compound: It contains two or more nonmetals that form covalent bonds.
When atoms share electrons, the bond is a covalent bond.
When two or more atoms share electrons, they form a molecule.
Lewis Structure
The shared electrons, or bonding pairs, are shown as two dots or a single line between atoms.
The nonbonding pairs of electrons, or lone pairs, are placed on the outside.
Double Bond: It occurs when two pairs of electrons are shared.
Triple Bond: It occurs when three pairs of electors are shared.
Double and triple bonds form when the number of valence electrons is not enough to complete the octets of all the atoms in the molecule.
6.6: Electronegativity and Bond Polarity
The electronegativity of an atom is its ability to attract the shared electrons in a chemical bond.
Nonmetals have higher electronegativities than metals because nonmetals have a greater attraction for electrons than metals.
Fluorine (F) has the highest electronegativity which has a value of 4.0.
Metal Cesium (Cs) has the lowest electronegativity which has a value of 0.7.
The noble gases have no electronegativity values because they do not typically form bonds.
Nonpolar Covalent Bonds: A bond between atoms with identical or very similar electronegativity values.
Polar Covalent Bond: Occurs when bonds are between atoms with different electronegativity values, and the electrons are shared unequally.
Dipole: A polar covalent bond that has a separation of charges.
6.7: Shapes and Polarity of Molecules
Valence shell electron-pair repulsion (VSEPR) theory: The electron groups are arranged as far apart as possible around the central atom to minimize the repulsion between their negative charges.
According to VSEPR theory, minimal repulsion occurs when two electron groups are on opposite sides of the central Carbon (C) atom.
This gives the Carbon Dioxide (CO2) molecule a linear electron-group geometry and a linear shape with a bond angle of 180°.
Minimal repulsion occurs when three electron groups are as far apart as possible around the central C atom, which gives 120° bond angles.
This type of electron-group geometry is trigonal planar.
When there are four atoms attached to four electron groups, the shape of the molecule is tetrahedral.
Molecular Shapes for a Central Atom with Two, Three, and Four Bonded Atoms

Polarity of Molecules
Nonpolar Molecule
All the bonds are nonpolar or the polar bonds cancel each other out.
It also occurs when polar bonds cancel each other because they are in a symmetrical arrangement.
Polar Molecules
One end of the molecule is more negatively charged than the other end.
It occurs when the dipoles from the individual polar bonds do not cancel each other.
6.8: Attractive Forces in Compounds
Dipole-Dipole Attractions: These are attractive forces that occur between the positive end of one molecule and the negative end of another.
Hydrogen Bonds: These occur between the partially positive hydrogen atom in one molecule and the partially negative nitrogen, oxygen, or fluorine atom in another molecule.
Polar molecules containing hydrogen atoms bonded to highly electronegative atoms of nitrogen, oxygen, or fluorine form especially strong dipole–dipole attractions.
Dispersion Forces: These are very weak attractions that occur between nonpolar molecules.
The electrons in a nonpolar covalent molecule are distributed symmetrically.
Electrons may accumulate more in one part of the molecule than another, which forms a temporary dipole.
 Knowt
Knowt
